
The XVIVO System (XPS™) is part of our ambition to provide our customers with solutions and systems that can improve the transplant process outcome, facilitate the work of the transplant team and enhance the long-term outcomes and quality of life for the transplant recipient.
The XPS™ is CE-marked and FDA approved for ex vivo lung perfusion of initially unacceptable donated lungs*.
The NOVEL Study – Establishing the safety and efficacy of the method
The NOVEL/NOVEL Extension trial was the first prospective, multi-center clinical trial in the world designed to evaluate the safety and efficacy of Ex-Vivo Lung Perfusion (EVLP) as a method to safely reassess good quality donor lungs that were previously unused or rejected for transplantation.
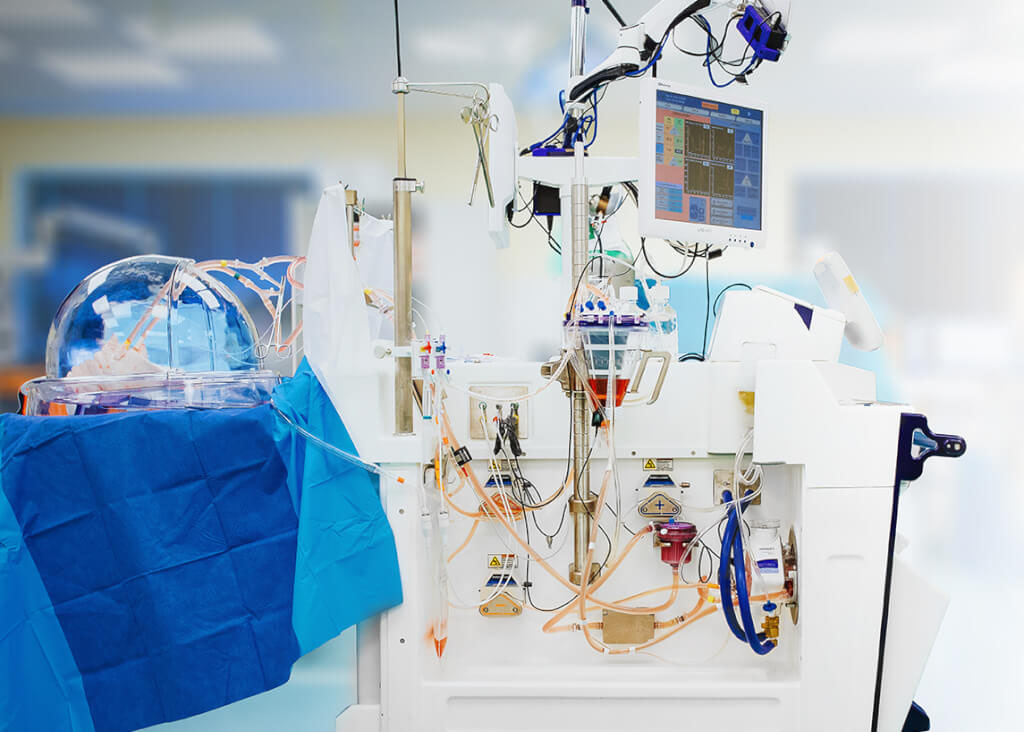

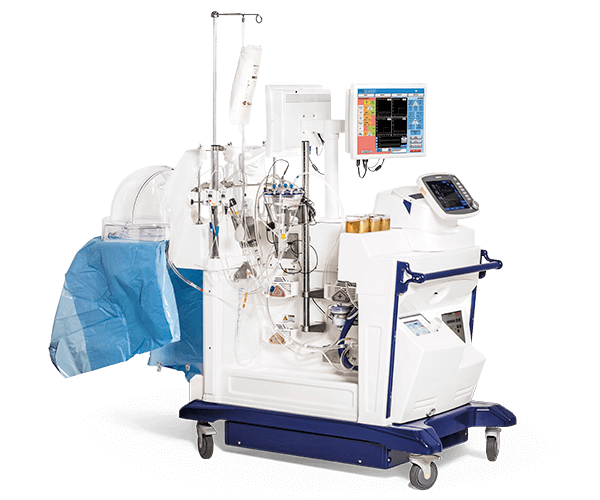
XPS™ is a fully integrated cardiac bypass system that includes all components. The XPS™ system is based on innovative technology from leading companies and includes a centrifugal pump (MAQUET CardioHelp), heater/cooler and ICU-ventilator (Hamilton).
The XPS™ is indicated for use in flushing and temporary continuous normothermic machine perfusion of initially unacceptable excised donor lungs during which time the ex vivo function of the lungs can be reassessed for transplantation.
This product is intended for use only by qualified medical professionals trained in the particular technique and/or surgical procedure to be performed.*
The XVIVO System (XPS™) is intended for flushing and temporary continuous normothermic machine perfusion of isolated lungs, during which time the function of the lungs can be assessed for transplantation. (for all other countries)
The system was developed together with clinicians with extensive experience and knowledge from normothermic EVLP using STEEN Solution™. Understanding the clinical needs and challenges transplant teams encounter in daily work, XPS™ facilitates the clinical decision making and offers a flexible comprehensive platform for EVLP.
XPS™ was developed to provide transplantation teams with a consistent and easy-to-use method of performing EVLP in the hospital. The objective was to develop an automated perfusion system that would allow for standardization of EVLP, without interfering with the need for clinical flexibility.
The donor lungs are transported according to individual standard protocol, in a cooler box from the procurement site. The donor lungs are flushed thoroughly in PERFADEX® Plus and then placed on the EVLP platform. After warming up according to a detailed protocol, it is up to the EVLP team to set preferred ventilation and perfusion strategies depending on the requirements of each donor lung. Integrated in-line perfusate gas monitors (PGM) enables for real-time trending of pH and pO2 during the entire EVLP procedure. The XPS™ also enables precise continuous trending of EVLP performance metrics.
XPS™ is specifically designed to facilitate X-ray while performing normothermic EVLP. If a mobile X-ray unit is not available, XPS™ may be transported at shorter distances within the hospital as it has a battery built-in that could support up to 20 min. This allows for simultaneous X-ray without interrupting the EVLP process.
Continuous data recording – evaluate for safety and control
The touch-display monitors and software allows trending the procedure and components as well as data capturing. The software captures and displays key performance data in real time. Parameters displayed are PVR, delta pO2 etc. Data can easily be downloaded via an USB port.
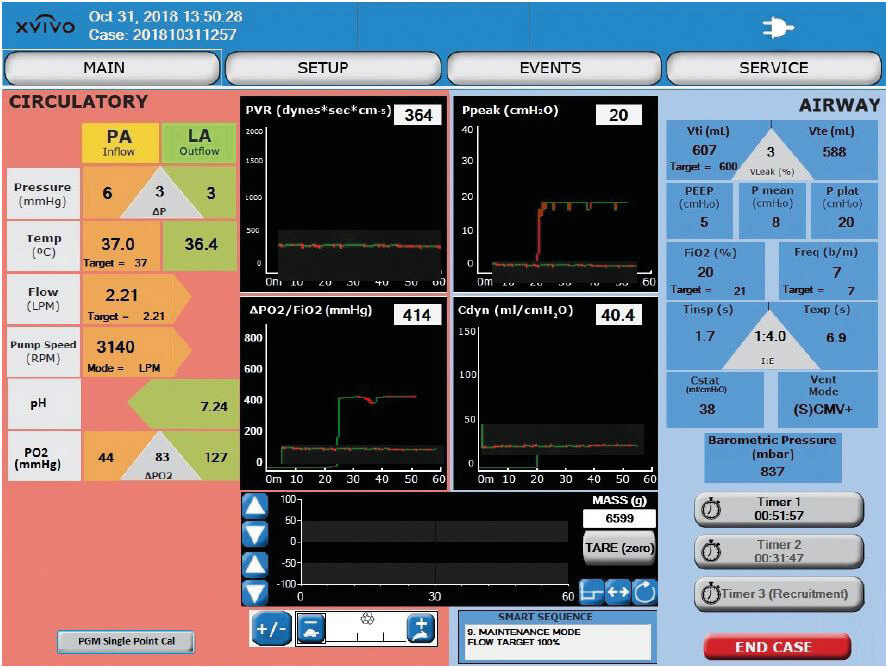
In-line Perfusate Gas Monitors (PGM) for real-time trending
The XPS™ is equipped with two in-line gas sensors, which enables real-time trending of pH and pO2 during EVLP.
The use of EVLP have after extensive experimental research been successfully transformed into clinical practice. Published reports from several centers show clinical outcomes equivalent to standard transplantation practices and clinical trials confirm these results.
Order: REF 19040 XPS™; 1 unit REF 19030 XPS™ (for US market only); 1 unit
Content: 1 unit. The XPS™ is a fully integrated off-the-shelf cardiac bypass system that includes components needed to safely run normothermic EVLP. The XPS™ system includes a centrifugal pump (MAQUET CardioHelp), heater/cooler and ICU-ventilator (Hamilton).
Storage: Store at room temperature
Intended use: XPS™ with STEEN™ Solution is indicated for use in flushing and temporary continuous normothermic machine perfusion of initially unacceptable excised donor lungs during which time the ex vivo function of the lungs can be reassessed for transplantation.
The XVIVO System (XPS™) is intended for flushing and temporary continuous normothermic machine perfusion of isolated lungs, during which time the function of the lungs can be assessed for transplantation. (For all other countries)
The system received the CE mark in March 2014 together with the accompanying disposable products. In April 2019, the XPS™ and disposable products received PMA approval by the US FDA and the system is now available for all customers in the US without any HDE restrictions.
XPS™ has been developed in contact with Toronto General Hospital and according to their successful clinical development of EVLP. All of the participating centers in the ongoing NOVEL trial in the US are using the XPS™ system. The XPS™ will make the EVLP procedure easier to perform, demand less time to set up and use fewer personnel than would a manually performed EVLP.
*In the US, the XPS™ with STEEN™ Solution is indicated for use in flushing and temporary continuous normothermic machine perfusion of initially unacceptable excised donor lungs during which time the ex vivo function of the lungs can be reassessed for transplantation.